Stereoselective synthesis of hydroxylated 3-aminoazepanes using a multi-bond forming, three-step tandem process†
Abstract
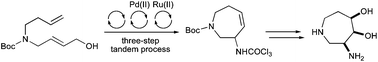
A multi-bond forming, three-step tandem process involving a palladium(II)-catalysed Overman rearrangement and a ring closing metathesis reaction has been utilised for the efficient synthesis of a 2,3,6,7-tetrahydro-3-amidoazepine. Substrate directed

 Please wait while we load your content...
Please wait while we load your content...