Preparation of enantioenriched iodinated pyrrolinones by iodocyclization of α-amino-ynones†
Abstract
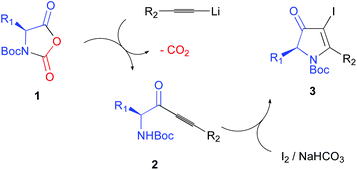
The unprecedented electrophilic iodo-mediated cyclization of α-amino-ynones afforded enantiomerically enriched β-iodopyrrolin-4-ones in excellent yields under mild conditions. The starting substituted α-amino-ynones were obtained from the chiral pool by selective mono-addition of an organolithium to optically pure N-protected carboxyanhydrides of

 Please wait while we load your content...
Please wait while we load your content...