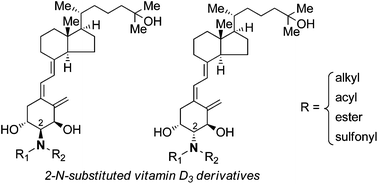
Binding of a series of novel 1α,25-dihydroxyvitamin D3 (1,25-VD3) derivatives, having a nitrogen-linked substituent at the 2α- or 2β-position of the A-ring (2-N-substituted compounds), with the vitamin D receptor (VDR) was investigated by means of computational docking studies. Selected compounds were synthesized by coupling A-ring synthons 6 and/or 7 with CD-ring-bearing bromomethylene 5 under Trost's conditions. The 2α- and 2β-stereoisomers of the A-ring synthons were synthesized from L-serine (8) as a single chiral source by installing vinyl and propargyl groups at opposite ends of the molecule. The activity of the obtained compounds was evaluated by means of a luciferase-based VDR transcriptional activity assay in NIH3T3 cells. Relatively small substituents incorporating a hydrogen-bonding donor, i.e., NHAc and NHMs, were effective for eliciting VDR transcriptional activity, and 2β-NHMs-1,25-VD3 (Xa) showed the highest activity, being more potent than 1,25-VD3. Derivatives with bulky substituents were inactive. These new insights into the structure–activity relationships of 1,25-VD3 derivatives may be helpful in separating the various biological activities of 1,25-VD3 and in generating novel therapeutic drug candidates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...