An approach to aliphatic 1,8-stereocontrol: diastereoselective syntheses of (±)-patulolide C and (±)-epipatulolide C†
Abstract
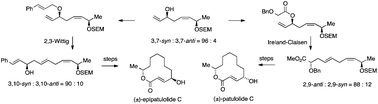
The tin(IV) bromide promoted reaction of 7-hydroxy-7-phenylhept-2-enyl(tributyl)stannane 11 with benzaldehyde gave a mixture of the epimeric 1,8-diphenyloct-3-ene-1,8-diols 12 and so indirect methods were developed for aliphatic 1,8-stereocontrol to complete diastereoselective syntheses of (±)-patulolide C 1 and (±)-epipatulolide C 40. (5Z)-3,7-syn-7-(2-Trimethylsilylethoxy)methoxyocta-1,5-dien-3-ol 17 was prepared from the tin(IV) chloride promoted reaction of 4-(2-trimethylsilylethoxy)methoxypent-2-enyl(tributyl)stannane 16 with acrolein (1,5-syn : 1,5-anti = 96 : 4). An Ireland–Claisen rearrangement of the corresponding benzoyloxyacetate 21 with in situ esterification of the resulting acid using trimethylsilyldiazomethane gave methyl (4E,7Z)-2,9-anti-2-benzyloxy-9-(2-trimethylsilylethoxy)methoxydeca-4,7-dienoate 22 together with 10–15% of its 2,9-syn-epimer 26, the 2,9-syn- : 2,9-anti-ratio depending on the conditions used. An 88 : 12 mixture of esters was taken through to the tert-butyldiphenylsilyl ether 38 of (±)-patulolide C 1 together with 6% of its epimer 39, by reduction, a Wittig homologation and deprotection/macrocyclisation. Following separation of the epimeric silyl ethers, deprotection of the major epimer 38 gave (±)-patulolide C 1. The success of 2,3-Wittig rearrangements of allyl ethers prepared from (5Z)-3,7-syn-7-(2-trimethylsilylethoxy)methoxyocta-1,5-dien-3-ol 17 was dependent on the substituents on the allyl ether. Best results were obtained using the pentadienyl ether 56 and the cinnamyl ether 49 that rearranged with >90 : 10 stereoselectivity in favour of (1E,5E,8Z)-3,10-syn-1-phenyl-10-(2-trimethylsilylethoxy)methoxyundeca-1,5,8-trien-3-ol 50. This product was taken through to the separable silyl ethers 38 and 39, ratio 7 : 93 by regioselective epoxidation and alkene reduction using diimide, followed by deoxygenation, ozonolysis, a Wittig homologation and selective deprotection/macrocyclisation. Deprotection of the major epimer 39 gave (±)-epipatulolide C 40.

 Please wait while we load your content...
Please wait while we load your content...