Essential reactive intermediates in nucleoside chemistry: cyclonucleoside cations†
Abstract
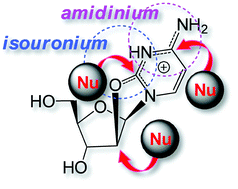
DFT-based modeling as well as experimental examination of model keto nucleosides have revealed that high susceptibility of these compounds to acids is due to formation of intermediate cyclonucleoside cations of low energy. Theoretically established chemical structures of these previously overlooked intermediates explain the reaction courses for a cluster of nucleoside reactions.

 Please wait while we load your content...
Please wait while we load your content...