Stereoselective synthesis of protected 3-amino-3,6-dideoxyaminosugars†
Abstract
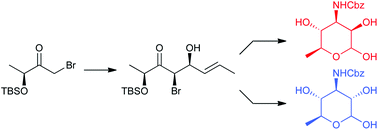
New syntheses of densely functionalized protected derivatives of 3-amino-3,6-dideoxyaminosugars have been accomplished in an efficient and straightforward manner. The key step of such approaches involves a highly stereoselective titanium-mediated aldol addition of a chiral α-bromo ketone, easily available from lactate

 Please wait while we load your content...
Please wait while we load your content...