Highly enantioselective Biginelli reaction catalyzed by SPINOL-phosphoric acids†
Abstract
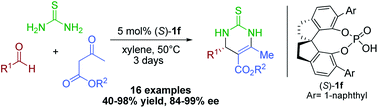
A highly enantioselective Biginelli reaction promoted by chiral spirocyclic SPINOL-phosphoric acids has been developed. Under the optimized conditions with 5 mol% catalyst loading, a wide range of optically active dihydropyrimidinethiones (DHPMs) were obtained in high yields (up to 98%) with good to excellent enantioselectivities (up to 99% ee). The synthetic utility of this method was demonstrated by the synthesis of chiral precursors of three drugs, including (S)-Monastrol, (S)-L-771688 and (S)-SQ 32926.
 Please wait while we load your content...
Please wait while we load your content...