Generation of molecular complexity from cyclooctatetraene using dienyliron and olefin metathesis methodology†
Abstract
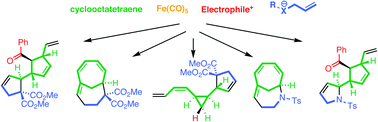
Transformation of the simple hydrocarbon cyclooctatetraene into a variety of polycyclic skeletons was achieved by sequential coordination to iron, reaction with electrophiles followed by allylated nucleophiles, decomplexation and
 Please wait while we load your content...
Please wait while we load your content...