Synthesis of a new family of acyclic nucleoside phosphonates, analogues of TPases transition states†
Abstract
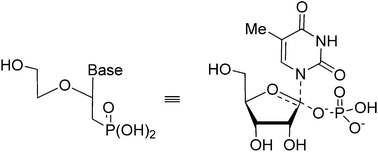
A 6-step procedure was developed for the synthesis of a new family of acyclic nucleoside
* Corresponding authors
a
AM2N, Institut Charles Gerhardt, UMR 5253, ENSCM, 8 rue de l'Ecole Normale, F-34296 Montpellier, France
E-mail:
david.virieux@enscm.fr
Fax: +33 (0)467 14 43 19
Tel: +33 (0)467 14 43 14
b Idenix Pharmaceuticals, Medicinal Chemistry Laboratory, Cap Gamma, 1682 rue de la Valsière, BP50001, 34189 Montpellier Cedex 4, France
c UMR5247 CNRS-UM1-UM2 (IBMM), Université Montpellier 2, 34095 Montpellier Cedex 5, France
A 6-step procedure was developed for the synthesis of a new family of acyclic nucleoside

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
B. Dayde, S. Benzaria, C. Pierra, G. Gosselin, D. Surleraux, J. Volle, J. Pirat and D. Virieux, Org. Biomol. Chem., 2012, 10, 3448 DOI: 10.1039/C2OB25131K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
