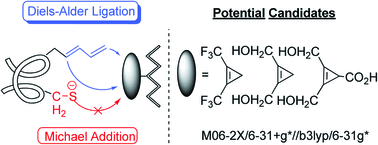
Bioorthogonal reactions are useful tools to gain insights into the structure, dynamics, and function of biomolecules in the field of chemical biology. Recently, the Diels–Alder reaction has become a promising and attractive procedure for ligation in bioorthogonal chemistry because of its higher rate and selectivity in water. However, a drawback of the previous Diels–Alder ligation is that the widely used maleimide moiety as a typical Michael acceptor can readily undergo Michael addition with nucleophiles in living systems. Thus, it is important to develop a nucleophile-tolerant Diels–Alder system in order to extend the scope of the application of Diels–Alder ligation. To solve this problem, we found that the theoretical protocol M06-2X/6-31+G(d)//B3LYP/6-31G(d) can accurately predict the activation free energies of Diels–Alder reactions with a precision of 1.4 kcal mol−1 by benchmarking the calculations against the 72 available experimental data. Subsequently, the electronic effect and ring-strain effect on the Diels–Alder reaction were studied to guide the design of the new dienophiles. The criteria of the design is that the designed Diels–Alder reaction should have a lower barrier than the Michael addition, while at the same time it should show a similar (or even higher) reactivity as compared to the maleimide-involving Diels–Alder ligation. Among the designed dienophiles, three substituted cyclopropenes (i.e. 1,2-bis(trifluoromethyl)-, 1,2-bis(hydroxylmethyl)- and 1,2-bis(hydroxylmethyl)-3-carboxylcyclopropenes) meet our requirements. These substituted cyclopropene analogs could be synthesized and they are thermodynamically stable. As a result, we propose that 1,2-bis(trifluoromethyl)-, 1,2-bis(hydroxylmethyl)- and 1,2-bis(hydroxylmethyl)-3-carboxylcyclopropenes may be potential candidates for efficient and selective Diels–Alder ligation in living systems.

 Please wait while we load your content...
Please wait while we load your content...