Syntheses, optical and intramolecular magnetic properties of mono- and di-radicals based on nitronyl-nitroxide and oxoverdazyl groups appended to 2,6-bispyrazolylpyridine cores†
Abstract
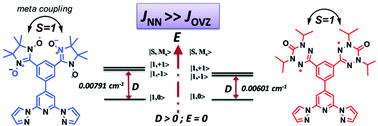
This paper presents the synthesis of a series of nitronyl-nitroxide (NN), oxoverdazyl (OVZ) based mono-, and bi-radicals attached to 4-phenyl-2,6-bispyrazolylpyridine coupling unit, their optical, electron spin resonance (ESR) spectroscopic studies and computational analysis. The ESR studies revealed that the axial zero-field splitting (zfs) parameter of the NN biradical (|D/hc| = 0.00719 cm−1) is larger than the OVZ biradical (|D/hc| = 0.00601 cm−1). Additionally both biradicals displayed forbidden half-field transitions (ΔMs = ±2; gav ∼ 4.01) at 170 K demonstrating their triplet nature. The cryogenic ESR measurements of the two biradicals showed a Curie magnetic behaviour of the ΔMs = ±2 signal intensities (χEPR) down to 4.2 K. A detailed comparative analysis of the strength of hyperfine coupling, spin density distribution, zfs and the spin–spin exchange coupling (J) of both NN and OVZ based biradicals showed that the ground state spin multiplicity of both biradicals is probably triplet (S = 1) or it is nearly degenerate singlet–triplet states with JNN ≫ JOVZ.

 Please wait while we load your content...
Please wait while we load your content...