Simple copper/TEMPO catalyzed aerobic dehydrogenation of benzylic amines and anilines†
Abstract
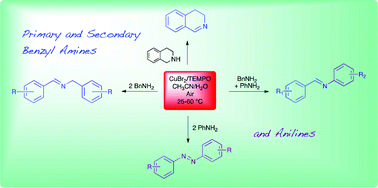
CuBr2 with 2,2,6,6-tetramethylpiperidyl-1-oxy (TEMPO) has been successfully employed for the aerobic oxidation of primary and secondary benzyl amines in aqueous

 Please wait while we load your content...
Please wait while we load your content...