Thiolation of symmetrical and unsymmetrical diketopiperazines†
Abstract
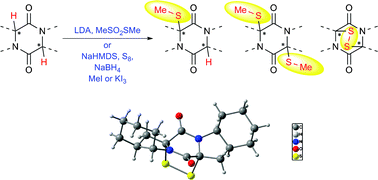
The introduction of sulfur units into a variety of symmetrical and unsymmetrical diketopiperazines (DKPs) is described. We investigated different thiolation methods utilizing several bases and electrophilic sulfur reagents, leading to monomethylthio-, bis(methylthio)-, and epithio-DKPs. Their formation proceeded diastereoselectively, facilitating the application in total syntheses of many thiodiketopiperazine (TDKP) natural products. Furthermore, possible side reactions as well as mechanistic studies and stereochemical structural assignments of the obtained products are given.

 Please wait while we load your content...
Please wait while we load your content...