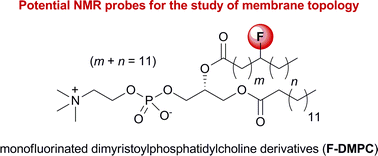
Synthesis and properties of monofluorinated dimyristoylphosphatidylcholine derivatives: Potential fluorinated probes for the study of membrane topology†
Abstract
The synthesis of three monofluorinated dimyristoylphosphatidylcholine derivatives (F-DMPC), with the fluorine atom located on the

 Please wait while we load your content...
Please wait while we load your content...