Triazacyclophane (TAC)-scaffolded histidine and aspartic acid residues as mimics of non-heme metalloenzyme active sites†
Abstract
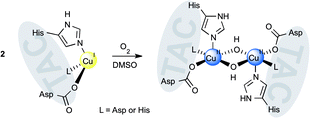
We describe the synthesis and coordination behaviour to copper(II) of two close structural triazacyclophane-based mimics of two often encountered

 Please wait while we load your content...
Please wait while we load your content...