Discovery of a potent and highly β1 specific proteasome inhibitor from a focused library of urea-containing peptide vinyl sulfones and peptide epoxyketones†
Abstract
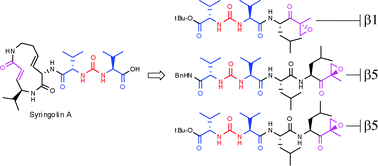
Syringolins, a class of natural products, potently and selectively inhibit the proteasome and show promising antitumour activity. To gain insight in the mode of action of syringolins, the ureido structural element present in syringolins is incorporated in

 Please wait while we load your content...
Please wait while we load your content...