Synthesis of enantioenriched γ-quaternary cycloheptenones using a combined allylic alkylation/Stork–Danheiser approach: preparation of mono-, bi-, and tricyclic systems†
Abstract
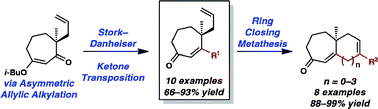
A general method for the synthesis of β-substituted and unsubstituted cycloheptenones bearing enantioenriched all-carbon γ-quaternary stereocenters is reported.

 Please wait while we load your content...
Please wait while we load your content...