Non-catalytic approach to the synthesis of partially reduced ‘S’ shaped dioxathia- and oxadithiahelicenes through base induced inter- and intramolecular C–C bond formation†
Abstract
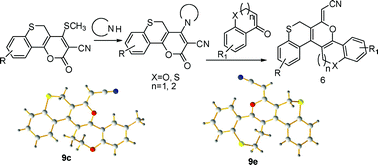
An efficient and convenient route for the construction of helical ‘S’ shaped dioxathia- and oxadithiahelicenes with oxygen and sulfur atoms located in the middle of the outer helix has been developed through base induced inter- and intramolecular C–C bond formation from the reaction of 4-sec-amino-2-oxo-2,5-dihydrothiochromeno[4,3-b]pyran-3-carbonitriles with 3,4-dihydro-2H-benzo[b]oxepin-5(2H)-ones,

 Please wait while we load your content...
Please wait while we load your content...