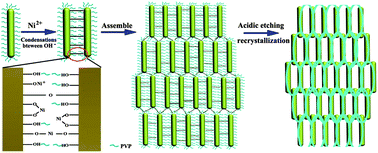
Uniform porous three-dimensional nanoarchitectures of α-Fe2O3 with high yield have been synthesized by a route based on a Ni2+/surfactant system. The obtained α-Fe2O3 has a flue-like porous morphology with a rough surface. What is more, spatially ordered nodes and meshes are presented in these networks. By adjusting the experimental parameters such as temperature, reaction time, proportion of Ni2+/PVP and types of cation, we can control the morphology of the samples well. A possible formation mechanism is proposed to explain the growth of the flue-like nanostructures. The obtained flue-like porous α-Fe2O3 has a large specific surface area of 88.82 m2 g−1. It exhibits significantly enhanced visible-light-driven photocatalytic performance in the degradation of methylene blue, compared with α-Fe2O3 nanoparticles. This work not only enriches the synthesis methods of porous architectures, but also facilitates the applications of α-Fe2O3 in the fields of water treatment, sunlight utilization and so forth.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...