Π-Bond maximization of graphene in hydrogen addition reactions†
Abstract
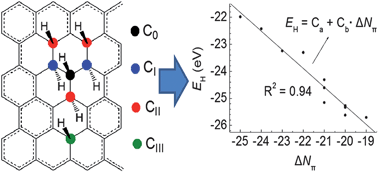
Thermodynamic stability of graphene hydrides increases in an approximately linear way with the numbers of π-bonds they contain. Thus, π-bond maximization is the primary driving force for hydrogen addition reactions of
- This article is part of the themed collection: Modelling of the nanoscale

 Please wait while we load your content...
Please wait while we load your content...