Tetraazaarenes by the ceramidonine approach†
Abstract
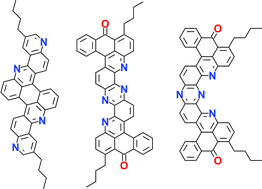
The synthesis of extended heteroarenesvia the acid-promoted dehydrocyclisation of arylamino-anthraquinones is examined as an approach to highly conjugated electron-acceptor materials and eventually to heterographene

 Please wait while we load your content...
Please wait while we load your content...