Blue and red shift hydrogen bonds in crystalline cobaltocinium complexes†
Abstract
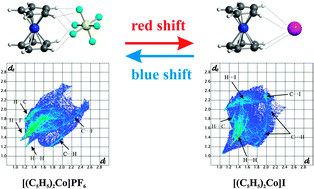
Typical hydrogen-bonded cobaltocinium salts of formula [Cp2Co+][A−] [with Cp = C5H5 and A = PF6 ( 1), AsF6 ( 2), SbF6 ( 3), I ( 4), I3 ( 5), Co(CN)6 ( 6), Co(CO)4 ( 7), Br3 ( 8), FeI4 ( 9) and HCl2 ( 10)] were studied by means of a combined structural, spectroscopic (IR, Raman, solid-state NMR) and theoretical approach. The solid-state vibrational spectra show blue or red shift hydrogen bond behavior depending on the anionic species, i.e. high- or low-frequency ν(CH) shift with respect to the solution value. The crystal structure of the new complex [Cp2Co+][SbF6−], a blue-shifted system, is reported while the [Cp2Co+][I−] complex, a red-shifted system disordered at room temperature, reveals a novel ordered polymorph at 150 K. The weak interactions (C⋯H, H⋯H, H⋯X, C⋯X) between cations and anions were analyzed by means of the Hirshfeld surfaces model, which permits their clear graphic visualization. HS fingerprint plots and normalized contact distances visually describe the difference between blue- and red-shifted complexes. Chemical shift tensors and shielding anisotropy values of the Cp carbon atoms, extracted from 13C CPMAS solid-state NMR spectra, allow the evaluation of Cp rotational motions which are related to the intermolecular contact extent. Finally, a DFT computational model is able to rationalize all the experimental data. It shows that the prevalence of one between two forces, that is, the attractive polarization of C–H bonds and the repulsive effect of electronic clouds, leads to the blue or red shift phenomenon.

 Please wait while we load your content...
Please wait while we load your content...