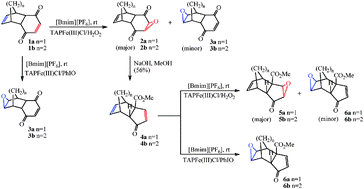
The oxygenation of substrates containing both electron rich and electron deficient olefins (1a–b) catalyzed by 5,10,15,20-tetraarylporphyrinatoiron(III) chlorides [TAPFe(III)Cl] with H2O2 gave epoxides at both electron rich and deficient olefin (2a–b, 3a–b), while with iodosyl benzene (PhIO) gave the epoxides only at electron rich olefin (3a–b) in imidazolium ionic liquids (ILs). Further reaction of 2a–b with sodium hydroxide in methanol gave 4a–b by base catalysed rearrangement of enedione epoxides. Similar reaction of 4a–b with H2O2 or PhIO gave the major epoxides of electron deficient olefins and electron rich olefins. The ferric peroxy anions (TAP-FeIII–OO−) are effective intermediates in the epoxidation of electron deficient olefins, whereas the high valent oxoferrylporphyrin π-cation radicals (TAP-FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O˙+) are involved in the epoxidation of electron rich olefins. The ILs provide the special microenvironments by the interactions of cations and anions, in which the generation of the active intermediates from TAPFe(III)Cl/[Bmim][PF6] and monooxygen donors could be accelerated significantly.
O˙+) are involved in the epoxidation of electron rich olefins. The ILs provide the special microenvironments by the interactions of cations and anions, in which the generation of the active intermediates from TAPFe(III)Cl/[Bmim][PF6] and monooxygen donors could be accelerated significantly.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O˙+) are involved in the
O˙+) are involved in the 

 Please wait while we load your content...
Please wait while we load your content...