Radical SAM enzymes in methylation and methylthiolation
Abstract
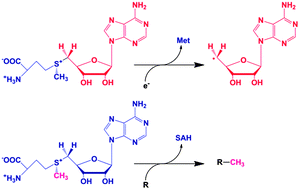
Radical S-adenosyl-L-methionine (SAM) enzymes are a large and diverse superfamily with functions ranging from enzyme
* Corresponding authors
a
Department of Chemistry and Biochemistry and the Astrobiology Biogeocatalysis Research Center, Montana State University, Bozeman, USA
E-mail:
jbroderick@chemistry.montana.edu
Tel: +1 406 994 6160
Radical S-adenosyl-L-methionine (SAM) enzymes are a large and diverse superfamily with functions ranging from enzyme

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
