Design, synthesis and biological activity evaluation of regioisomeric spiro-(indoline-isoxazolidines) in the inhibition of TNF-α-induced ICAM-1 expression on human endothelial cells†
Abstract
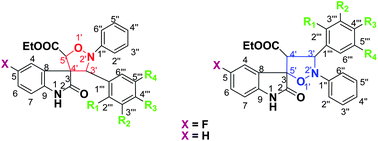
Different regioisomeric spiro-(indoline-isoxazolidines) have been synthesized by the microwave mediated cycloaddition reaction between ethyl(5-fluoro-3-indolylidene)-acetate/ethyl(3-indolylidene)-acetate and the substituted α,N-diphenylnitrones. These compounds were screened for their anti-inflammatory activities through inhibition of TNF-α-induced ICAM-1 expression on human endothelial cells. Spiro-(indoline-isoxazolidine) 9p was found to be the most potent compound in inhibiting the ICAM-1 expression. Furthermore, compound 9p significantly inhibited the adhesion of neutrophils to the endothelium in a concentration-dependent manner and also blocked the translocation of NF-κBp65 subunit from the cytoplasm to the nucleus. We believe that compound 9p could be useful in developing better anti-inflammatory molecules for various inflammatory diseases where NF-κB plays a key role.

 Please wait while we load your content...
Please wait while we load your content...