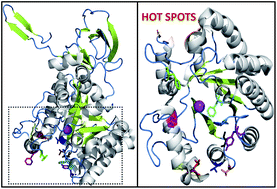
Suicide gene therapy (SGT) has emerged as a technique for the treatment of cancer, wherein a gene is consigned to cancer cells, translation of which results in an enzyme exhibiting the potential to convert a nontoxic prodrug to a toxic drug and thereby triggering cancer cell death. The bacterial cytosine deaminase (bCD)/5-fluorocytosine (5-FC) approach is an attractive method of gene therapy because of the heightened bystander effect, but the inefficient binding of the prodrug 5-FC, limits its applicability. Herein, we designed mutants of E. coli cytosine deaminase to enhance its binding affinity towards the prodrug 5-FC. In silico site directed mutagenesis (SDM) was performed to construct novel mutants of bCD having enhanced binding affinity towards the prodrug than its actual substrate cytosine. The in silico results offered F186W, F82C, S126N and R91T mutants which showed efficient binding towards 5-FC as compared to cytosine; whereas, the mutant S126R bound only with 5-FC. Based on the in silico results, bCD mutants were developed through in vitro SDM. Furthermore, the mutant proteins were overexpressed, purified and their enzymatic activity toward cytosine and 5-FC were investigated. The functional characterization of these mutants revealed that the engineered proteins S126R, F186W and F82C possess enhanced catalytic efficiency towards 5-FC relative to the wild-type (WT) enzyme and can potentially be more effective for SGT.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...