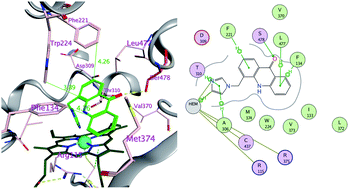
Two positional isomers with the general formula 1H-imidazol-1-ylmethylacridin-9(10H)-one were synthesized (5, 6) and evaluated for their inhibitory activity versus CYP11B1, CYP11B2, CYP17 and CYP19. Compound 5 was more effective than letrozole in inhibiting CYP19 (aromatase). Interestingly, compound 5 also inhibited CYP11B1 with an IC50 of 21.2 nM. On the other hand compound 6 was almost completely inactive against all CYPs; this indicates that the positioning and spatial orientation of the imidazolylmethyl moiety is of paramount importance to activity. Sequence alignment of the four steroidogenic CYP enzymes and docking studies with 5, 6 and letrozole supported this finding and suggested Ser478 to be an essential residue for both inhibition and selectivity. These novel compounds may have benefits for the treatment of Cushing's syndrome, hypertension, congestive heart failure and myocardial fibrosis and breast cancer.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...