l-Penicillamine is a mechanism-based inhibitor of serine palmitoyltransferase by forming a pyridoxal-5′-phosphate-thiazolidine adduct†‡
Abstract
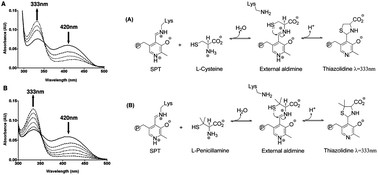
Serine palmitoyltransferase (SPT) catalyses the first committed step of de novo sphingolipid biosynthesis. The bacterial SPT homologue from Sphingomonas paucimobilis is a homodimeric enzyme that contains an essential pyridoxal-5′-phosphate (PLP) cofactor bound to each subunit. Inhibitors of SPT are useful blockers of sphingolipid biosynthesis. Here we use UV-vis spectroscopy, enzyme kinetics and mass spectrometry to investigate inhibition of SPT by penicillamine (Pen), a drug with a range of useful medicinal applications.

 Please wait while we load your content...
Please wait while we load your content...