Molecular dynamics and free energy studies on Aurora kinase A and its mutant bound with MLN8054: insight into molecular mechanism of subtype selectivity†
Abstract
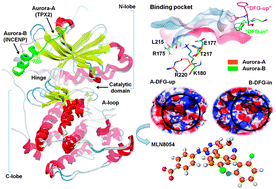
Because of the high conservation of ATP-binding sites in kinases, the quest for selective kinase inhibitors has been increasingly urgent in recent years. The Aurora kinase family represents attractive targets in cancer therapy and several small molecule inhibitors targeting Aurora kinases are undergoing clinical trials. Among them, MLN8054 has been proved to be a selective Aurora-A inhibitor, and is currently being evaluated in a phase I trial for patients with advanced solid tumors. But the detailed selectivity mechanism of MLN8054 towards Aurora-A over Aurora-B is still not resolved. In the present work, this selectivity mechanism was investigated using molecular dynamics simulations and binding free energy calculations. The predicted binding conformations and binding affinities of MLN8054 to Aurora-A and its mutant that mimics Aurora-B suggest that there exists stronger interaction between MLN8054 and Aurora-A through an induced DFG-up conformation. Further analyses can provide some information about the structural basis for the selectivity mechanism. Binding of MLN8054 to Aurora-A induces the conformation of the activation loop to adopt an unusual DFG-up conformation and opens the hydrophobic pocket of the active site, thus increasing the interaction between MLN8054 and the residue Val279. The residue Glu177 in Aurora-B displays electrostatic repulsion with MLN8054, while the corresponding Thr217 in Aurora-A has favorable interactions with MLN8054. The conformation change and the difference between the binding pockets for Aurora-A and B are key factors responsible for the selectivity. The results could be helpful for the rational design of selective inhibitors of Aurora-A kinase.
 Please wait while we load your content...
Please wait while we load your content...