Forced homodimerization of the c-Fos leucine zipper in designed bHLHZ-like hybrid proteins MaxbHLH-Fos and ArntbHLH-Fos†
Abstract
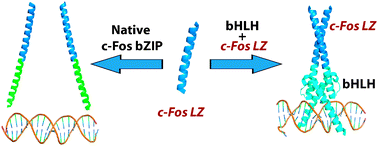
Although the c-Fos leucine zipper (LZ) does not form a homodimer in its native basic region/leucine zipper (bZIP) structure, we found that it is capable of homodimerization and promoting protein folding in engineered basic region/helix–loop–helix/leucine zipper (bHLHZ) hybrid proteins MaxbHLH-Fos and ArntbHLH-Fos, in which the bHLH subdomains of Max and Arnt are fused to the c-Fos LZ. By using the in vivo yeast one-hybrid system and in vitro circular dichroism and quantitative fluorescence anisotropy, we demonstrated that attachment of the c-Fos LZ to the otherwise unstructured MaxbHLH resulted in a hybrid bHLHZ-like protein now competent for homodimerization and DNA binding at the cognate E-box site, CACGTG. In ArntbHLH-Fos, the c-Fos LZ promoted proper folding of the HLH structure, although unlike MaxbHLH, ArntbHLH alone is capable of homodimerization and DNA binding. In addition, by comparing the E-box binding and secondary structures of MaxbHLH-Fos and two derivatives containing targeted mutations in the c-Fos LZ, we found that cooperative communication exists between the bHLH and LZ: proper folding of the four-helix bundle in the HLH region could be induced by the c-Fos LZ, and the HLH dimerization region could force homodimerization of the c-Fos LZ. These results demonstrate that although intrinsically unfavorable, the c-Fos LZ can homodimerize, demonstrating that the same c-Fos LZ element can yield orthogonal differences in structure and/or DNA-binding function within different transcription factor families, including the bZIP and bHLHZ.

 Please wait while we load your content...
Please wait while we load your content...