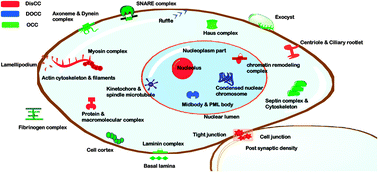
Intrinsic disorder in proteins has been explored to study lack of structure–function aspects of many proteins. The current study focuses on coiled coils which are often linked to intrinsic disorder. We present a sequence level analysis of human coiled coils to find out if this is universally true for all coiled coils. When annotated coiled-coil regions were collected from UniProt and investigated with disorder prediction tools namely—IUPred and DISpro, three patterns were commonly observed—disordered coiled coils (DisCCs), ordered coiled coils (OCCs) and the last one having a disordered region outside the coiled-coil region (DOCCs). Differential enrichment in the gene ontology was seen in these three categories. We found that OCCs are enriched in structural components of the extracellular space including the fibrinogen complex and laminin complex. On the contrary, DisCCs were found to be exclusively over-represented in proteins involved in actin filament, lamellipodium, cell junction, macromolecule complexes, ciliary rootlet and nucleolus. DOCCs are found to be associated with many regulatory and adaptor functions including positive regulation of calcium ion transport via store-operated calcium channel activity, cytoskeletal adaptor activity etc. Other than the GO-based analysis, sequence level analysis showed that disordered coiled-coil regions bear a high proportion of low-complexity regions as compared to ordered coiled coils. The former also has a higher probability of forming a dimer as compared to the ordered counterpart. Our study shows that the in silico approach of mapping of disorder in or around coiled coils in other biological systems or organisms can be applied to understand and rationalize the mode of action of these dynamic motifs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...