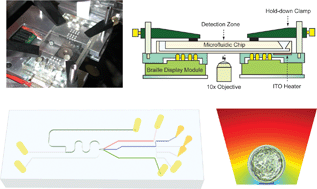
We report a computerized microfluidic real time embryo culture and assay device that can perform automated periodic analyses of embryo metabolism. This automated program uses a modified “gated injection” scheme (sample injection, reagent mixing, enzyme reaction of 15 min incubation, and sample detection) to sequentially measure fluorescence from sample, reference, and background (without any analyte) every hour. Measurements assessed with reference solutions demonstrated the stability of these microfluidic measurements over a 24 h period. Furthermore, this system was able to measure time dependent nutrient consumption by single or multiple (10) live mouse blastocyst-stage embryos with pmol h−1 sensitivity. Mechanical deformation-based microfluidic actuation created by computerized movement of Braille pins enables automated fluid pumping and valving sequences without unwanted gravity-driven backflow or exposure to electrical fields as would be required in electrokinetic schemes. The convenient, non-invasive, and automated nature of these assays open the way for the development of integrated microfluidic platforms for practical single embryo culture and real time biochemical analysis to assess embryo viability and select embryos with the greatest implantation potential, thus improving success in clinical assisted reproductive technology laboratories.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...