Understanding the stability of fluorosulfate Li-ion battery cathode materials: a thermochemical study of LiFe1−xMnxSO4F (0 ≤ x ≤ 1) polymorphs
Abstract
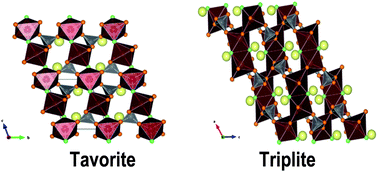
Isothermal acid solution calorimetry was employed to investigate the relative thermochemical stabilities of two polymorphs of the LiFe1−xMnxSO4F (0 ≤ x ≤ 1) solid solution series: triplite and tavorite. These compounds have shown promise as lithium-ion battery cathodes, and a fuller understanding of their thermodynamics will aid in synthesis and their practical application. The linear energetic trends among triplites and tavorites indicate greater stabilization of each of these structures from the binary components with increase in manganese content and suggest a negligible heat of mixing of Fe and Mn ions. The tavorite phase, formed for x < 0.2, appears energetically more stable than the triplite. The formation of the disordered triplite structure appears to be entropy driven, and the factors that increase the disorder of the system (e.g. rapid phase formation) favor the triplite structure. Further, the free energy change associated with the tavorite to triplite transformation obtained by calculating configurational entropies using measured enthalpies was almost zero (−1.3 ± 0.8 kJ mol−1) at ambient temperature but becomes exothermic at 500 °C (−4.3 ± 0.8 to −6.8 ± 0.8 kJ mol−1). This suggests that both tavorite and triplite (with random cation distribution) are equally stable at ambient temperature but the tavorite to triplite transformation is thermodynamically favored at high temperatures because of entropy.
- This article is part of the themed collection: Nanomaterials for Energy Conversion and Storage

 Please wait while we load your content...
Please wait while we load your content...