Enhanced anhydrous proton conduction in binary mixtures of 1H-imidazole–1H-1,2,3-triazole based compounds†
Abstract
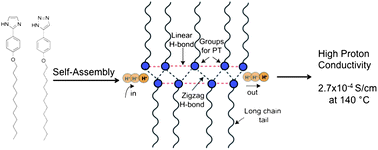
What is the impact of mixing two proton-conducting heterocycles on proton conductivity? Herein we answer this question through our investigations on two linear rod-like compounds 2-(4-(dodecyloxy)phenyl)-1H-imidazole (4) and 5-(4-(dodecyloxy)phenyl)-1H-1,2,3-triazole (10). We have found that mixtures of molecules 4 and 10 at certain compositions show enhanced proton conductivity compared to their pure components. We attribute the increased conductivity in these materials to the increased charge density due to facile co-ionization and increased mobility due to the incorporation of long alkyl chains, which prevent crystallization of protogenic groups while maintaining the required hydrogen bonded network. Our results suggest a new strategy for enhancing intrinsic proton conductivity in heterocyclic systems.

 Please wait while we load your content...
Please wait while we load your content...