Olivine LiCoPO4–carbon composite showing high rechargeable capacity†
Abstract
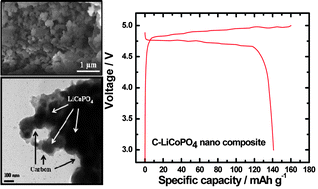
A LiCoPO4 positive electrode material with an extremely high discharge capacity, 145 mA h (g-phosphate)−1, is reported. Seeking high capacity, we examined three kinds of precursors, Co3O4, Co3(PO4)2·2H2O, and NH4CoPO4·H2O. In combination with a thermal gravimetric study, we found that simple the dehydration of the first two precursors is related to the formation of LiCoPO4–acetylene black carbon composites (hereafter referred as C–LiCoPO4). Meanwhile, the formation of the C–LiCoPO4 composite is somewhat different. That is, generation of NH3 gas and dehydration of the NH4CoPO4·H2O precursor occurs spontaneously, and the NH3, which decomposes to N2 and H2 gases, provides a more reductive environment during calcination, leaving a small quantity of metallic Co nanoparticles (<10 nm). Distribution of the added acetylene black carbon network is important for proper electron transfer, resulting in good rate capability and capacity retention at 25 °C and 55 °C, which has never been reported.

 Please wait while we load your content...
Please wait while we load your content...