Highly selective CO2/CH4 gas uptake by a halogen-decorated borazine-linked polymer†
Abstract
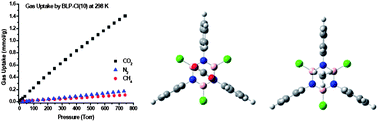
A new borazine-linked polymer featuring chlorine-decorated cavities, BLP-10(Cl), has been prepared by the thermal decomposition of benzidine–boron trichloride in toluene under refluxing conditions. BLP-10(Cl) exhibits a moderate Langmuir surface area (1308 m2 g−1) and one of the highest CO2/CH4 selectivities (28.3) by porous materials at 1.0 bar and 273 K. Computational studies revealed that H2, CO2, and CH4 interact more favorably with the borazine unit with isosteric heats of adsorption of 7.46, 28.3, and 20.2 kJ mol−1, respectively. These values are in good agreement with experimental data collected by using the virial method. Results from this study suggest that including highly polarizable and halogenated building units into the framework of porous architectures can significantly enhance their performance in gas separation applications.

 Please wait while we load your content...
Please wait while we load your content...