Facile strategy for synthesis of mesoporous crystalline γ-alumina by partially hydrolyzing aluminum nitrate solution
Abstract
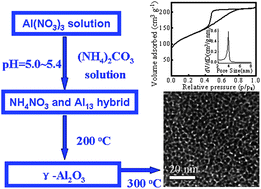
The facile synthesis of mesoporous γ-alumina is developed through the partial hydrolysis of Al(NO3)3 aqueous solution with (NH4)2CO3 without organic surfactants. In this synthesis, the stable NH4NO3/Al species (AN/Al) hybrid containing Keggin-Al13 polycations is first prepared, which is the key for the successful formation of mesoporous γ-alumina. XRD, 27Al MAS NMR, TEM, and N2 adsorption and desorption results demonstrate that the as-prepared AN/Al hybrid can be transformed to γ-alumina by treatment at 200 °C and exhibit a wormhole-like mesoporous structure with large surface area up to ∼450 m2 g−1, pore volume of ∼0.3 cm3 g−1 and narrow pore size distribution peaked at ∼3.9 nm after completely removing NH4NO3 at 300 °C. The obtained mesoporous γ-aluminas have high thermal stabilities up to 900 °C and excellent hydrothermal stability. The investigation shows that the synergetic effect of NH4NO3 and Al13 species promotes crystallization of Al species to γ-alumina, which may have a unique mechanism distinct from the mesoporous aluminas reported previously. CO2 adsorption measurements indicate that these mesoporous γ-aluminas have a much higher CO2 adsorption capacity than ordered mesoporous alumina synthesized by the surfactant-templating method and conventional γ-alumina derived from aluminum oxyhydroxides. We believe that this research should be useful for providing new insights into the transformation of transition alumina phases and for synthesizing mesoporous γ-alumina with promising properties for various chemical applications.

 Please wait while we load your content...
Please wait while we load your content...