Observation of the highest Mn3+/Mn2+ redox potential of 4.45 V in a Li2MnP2O7 pyrophosphate cathode
Abstract
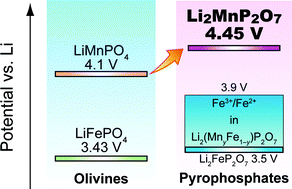
By exploring the pyrophosphate chemistry for rechargeable Li-ion batteries, we report the synthesis and electrochemical characterization of a Li2MnP2O7 cathode. Easily prepared by conventional solid-state synthesis (at 600 °C), the Li2MnP2O7 displays an Mn3+/Mn2+ redox potential centered at 4.45 V versus lithium. It has registered the highest voltage ever obtained for the Mn3+/Mn2+ redox couple in any Mn-based cathode material. Following the pristine and partially (Mn) substituted Li2FeP2O7 (3.5–3.9 V vs. Li) and Li2CoP2O7 (4.9 V vs. Li), which show the highest Fe3+/Fe2+ and Co3+/Co2+ redox potentials among the known cathode compounds, the highest Mn3+/Mn2+ redox voltage (ca. 4.45 V) in Li2MnP2O7 establishes ‘pyrophosphates’ as a novel family displaying the highest M3+/M2+ redox potentials among all polyanionic compounds. Fundamental study of these pyrophosphates can provide useful insights into design of high-voltage cathode materials.

 Please wait while we load your content...
Please wait while we load your content...