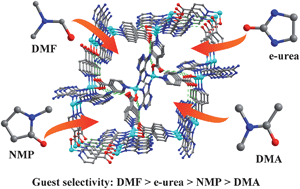
Presented here is the unusual guest selectivity observed in the self-assembly process of a porous tetrahedral imidazolate framework material [Zn(ad)(int)] (TIF-1, ad = adeninate, int = isonictinate). Four distinct solvent molecules, such as N,N-dimethylformamide (DMF), ethyleneurea (e-urea), N-methy1-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMA), can be trapped in the same in situ generated TIF-1 framework, respectively, which are identified by single-crystal X-ray diffraction. Complicated mixed solvent systems are investigated to understand the guest selectivity of TIF-1. The results reveal TIF-1 has strict guest selectivity following the sequence DMF > e-urea > NMP > DMA. Moreover, the activated TIF-1 after methanol-exchange exhibits high H2 and CO2 adsorption capacity and remarkable selectivity of CO2 over N2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...