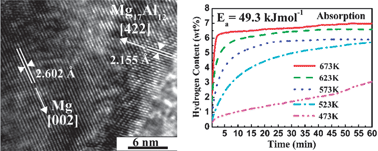
In order to improve the hydrogen storage properties of Mg, Mg@Mg17Al12 ultrafine particles (UFPs) with 7, 22 and 27 at% Al have been successfully prepared by a hydrogen plasma–metal reaction (HPMR) approach. These UFPs are nearly spherical in shape with an average size of about 150 nm. The Mg particle core is a single crystal, and the Mg17Al12 particle shell of 2–5 nm thickness effectively suppresses the formation of MgO. The formation mechanism of the core–shell structure is interpreted in terms of Mg–Al phase transformation. The Mg17Al12 shell disproportionates into MgH2 and Al upon hydrogenation, and is recovered after hydrogen release. The morphology and size of these UFPs are not obviously changed during the sorption cycle, whereas the Mg particle core changes from single crystal into the polycrystalline form of 2–4 nm size. The hydrogen sorption kinetics and storage capacity of the Mg@Mg17Al12 UFPs decreased with increasing Al content. Mg–7 at.% Al can absorb 5.7 wt% H2 at 523 K and 7.0 wt% H2 at 673 K. It can release 6.0 wt% H2 within 30 minutes at 623 K and 6.2 wt% H2 within 3 minutes at 673 K. The catalytic effect and oxidation resistance of the Mg17Al12 shell, and the nanostructure of the Mg core accelerate the hydrogen diffusion, with low hydrogen absorption and desorption activation energies of 49.3 and 105.5 kJ mol−1, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...