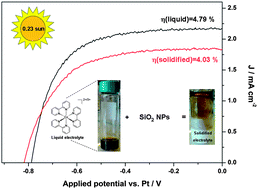
An innovative solidified redox electrolyte for dye-sensitized solar cells (DSCs) was prepared by incorporating amorphous silica nanoparticles in a liquid system containing the Co2+/Co3+ shuttle dissolved in methoxypropionitrile; the novel material was characterized by Raman spectroscopy and its optical properties were examined by UV-Vis spectroscopy. Electrochemical properties (diffusion, conductivity, electrolyte compatibility with the cathode) were studied in symmetrical thin layer cells using polarization and electrochemical impedance spectroscopy (EIS) measurements. Quasi-solid DSCs were fabricated by incorporating this electrolyte and attained an efficiency of about 2.6% under 1 sun (under 1000 W m−2) AM1.5G illumination, corresponding to 73% of that gained by the reference liquid electrolyte; the cell efficiency depends on the light illumination conditions, significantly increased up to more than 4% under 0.23 sun. The solar cells were further characterized by EIS and intensity modulated photocurrent spectroscopy (IMPS) to investigate charge transport and recombination dynamics, possible conduction band edge shifts, alterations of the interfacial and ohmic resistances, and variations of the diffusion rate through the photoelectrode. It was confirmed that the solidified electrolyte-based DSC presented a slightly enhanced photopotential in comparison with that of the liquid electrolyte due to a negative TiO2 conduction band edge shift upon contact with the electrolyte, accompanied with a decreased photocurrent mainly stemming from the restricted diffusion of the Co3+ species through the pores of the TiO2 photoelectrode.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...