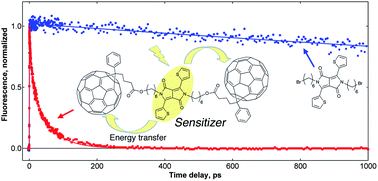
In an effort to develop new materials for organic solar cell applications, we have synthesized triads of 3,6-dithien-2-yl-2,5-dialkylpyrrolo[3,4-c]pyrrole-1,4-dione (DTDPP) covalently linked at the nitrogen positions to two [6,6]-phenyl-C61-butyric acid ester (PCB) units via alkyl chains of different lengths. We present here the excited-state properties of the compounds in solution, as investigated by (time-resolved) spectroscopy. The absorption spectra of the triads are the composite of the ones recorded with the separate fullerene and DTDPP parent molecules, indicating weak electronic coupling between the sub-units. However, the fluorescence quantum yield drops from 74% in pure DTDPP to <1% in the triads, in both polar o-dichlorobenzene (DCB) and non-polar toluene (TOL). According to the energy levels determined by cyclic voltammetry for the parent compounds, charge separation (CS) or excitation energy transfer (EET) could be responsible for the quenching. However, femtosecond-resolved transient absorption (TA) measurements revealed the quenching mechanism to be highly efficient EET from the DTDPP to the PCB moieties. Ultrafast fluorescence spectroscopy showed multiphasic EET dynamics, due to different molecular conformations induced by the flexibility of the alkyl linkers, with time constants ranging from the sub-picosecond to the 100–150 ps scale. The DTDPP chromophore thus acts as a sensitizer (or light-absorbing antenna) to channel light towards the fullerenes, which have low absorbance in the visible range. The ultrafast time scale of the EET leading to fast population of the PCB singlet excited state is particularly interesting for potential use of the systems to increase light harvesting in photovoltaic devices containing fullerenes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...