Electrical power and hydrogen production from a photo-fuel cell using formic acid and other single-carbon organics†
Abstract
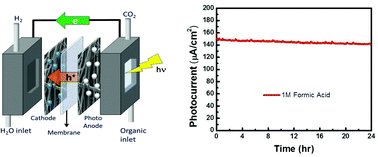
A photoelectrochemical cell based on a TiO2 photoanode, a Nafion membrane, and a platinum cathode was used to photo-oxidize formic acid to produce high-energy electrons, which were then used to produce H2. Using AM1.5 simulated sunlight, the cell had a photocurrent of 150 μA cm−2 and produced 60 μL h−1 cm−2 (88% faradaic efficiency). Polarization curves were taken and the cell produced a maximum power of 30 μW h−1 cm−2. The cell temperature was varied and the open circuit potential showed a trend consistent with Nernstian behavior while the polarization curves shifted slightly. To study effects of different organics, open circuit measurements and polarization curves were taken for formaldehyde and methanol in addition to formic acid. Though the three single-carbon organics have quite similar oxidation mechanisms, the photoelectrochemical behavior of methanol varied significantly from that of both formaldehyde and formic acid. Possible reasons for the differences are discussed.

 Please wait while we load your content...
Please wait while we load your content...