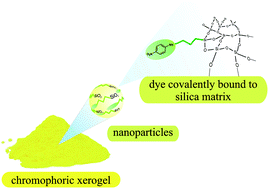
An advanced procedure for the one-pot synthesis of organic–inorganic hybrid materials via combination of sol–gel process and nucleophilic aromatic substitution reaction (SNAr) of 4-fluoronitrobenzene and 3-aminopropyltrimethoxysilane is described. With this advanced procedure both SNAr-reaction and sol–gel process can be accomplished in the same reaction vessel due to the sol–gel precursor tetraethoxysilane (TEOS) acting as solvent during the first reaction step. Via extensive NMR spectroscopic studies it is proven for the first time that—contrary to common belief—hydrogen fluoride (HF), which is formed as a by-product in the SNAr-reaction, is not trapped by any bases present but is rather trapped by both of the silane species and serves as a catalyst during the subsequent sol–gel process. The chromophoric system of the resulting xerogels is well protected against aggressive chemicals, i.e. strong acids, by the silica matrix, which predestines these materials for pigment applications. Given that a high chromophore content is highly desirable in these applications, we show that the chromophore content of the final xerogel can be varied by modification of the organosilicon precursor : TEOS ratio or by using trialkoxy-silanes bearing two or three amino functions, whereby the latter option is more favourable. Monodisperse core–shell particles with identical chromophore content but consisting of a pure silica-core and a p-nitroaniline functionalized shell with a diameter of about 200 nm can also be prepared using this advanced procedure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...