Glycation of plasma proteins in type II diabetes lowers the non-covalent interaction affinities for dietary polyphenols
Abstract
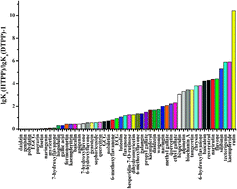
Diabetes is characterized by an elevated level of
* Corresponding authors
a
Department of Chemistry, Central South University, Changsha 410083, P. R. China
E-mail:
xqchen@mail.csu.edu.cn
Tel: +86 (731)8830833
b
Department of Biology, College of Life & Environment Science, Shanghai Normal University, 100 Guilin Rd, Shanghai 200234, PR China
E-mail:
jianboxiao1979@shnu.edu.cn
Fax: +86 (21)64321291
Tel: +86 13611600163
Diabetes is characterized by an elevated level of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
