Zinc phthalocyanine with PEG-400 as a recyclable catalytic system for selective reduction of aromatic nitro compounds†‡
Abstract
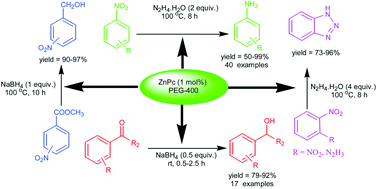
Zinc phthalocyanine with PEG-400 was established as a catalytic system for chemo and regioselective reduction of aromatic nitro compounds to corresponding amines. A large range of reducible functional groups such as acid, amide, ester, halogen, lactone, nitrile, N-benzyl, O-benzyl, hydroxy and heterocycles were well tolerated. Direct synthesis of benzotriazole from O-dinitrobenzene was achieved for the first time. The present catalytic system was successfully employed for the reduction of carbonyl and ester compounds to corresponding alcohols and reductive amination of benzaldehydes with primary amines to form corresponding secondary amines. Remarkable advantages of the present catalytic method include low loading of metal, avoidance of toxic ligands and high isolated yields. The catalyst was recyclable up to four times without any loss of selectivity and activity.

 Please wait while we load your content...
Please wait while we load your content...