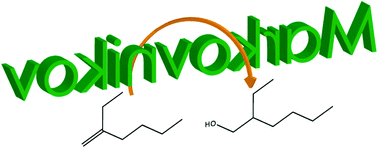
An efficient method for the formal anti-Markovnikov hydration of 1,1-disubstituted alkenes has been developed. The utility of the process has been demonstrated by conversion of bio-derived butene oligomers into primary alcohols through initial oxidation to vicinal acetoxy-alcohols, diols, or diacetates, followed by selective dehydration/tautomerization of the diols, and hydrogenation of the intermediary aldehydes. This approach allows for the isolation of important industrial plasticizer alcohols from a renewable source. In a broader context, this pathway, which can be conducted with sustainable, conventional reagents under mild conditions, represents a unique alternative to hydroboration for a challenging subset of hindered olefins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...