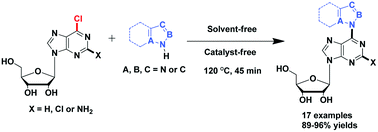
Synthesis of C6-azolyl purine nucleosides via C–N coupling reaction of unprotected 6-chloropurine nucleosides and N-heterocycles under catalyst- and solvent-free conditions†
Abstract
C6-azolyl purine nucleoside products are prepared in high yields under

 Please wait while we load your content...
Please wait while we load your content...