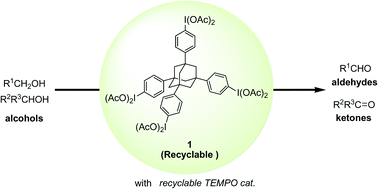
Using a recyclable hypervalent iodine reagent 1, the authors have constructed versatile and green methods for the hypervalent iodine and nitroxyl radical-mediated selective oxidation of alcohols to aldehydes and ketones. The recyclable reagent 1 having a unique tetraphenyladamantane structure exhibited almost the same reactivity as the ordinary reagent, phenyliodine diacetate (PIDA), in the hypervalent iodine(III)/2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO)-mediated oxidation. For recycling, the reagent 1 could be nearly quantitatively recovered as the reduced tetraiodide 2 by filtration after reaction completion, utilizing the insolubility of the formed 2 in a polar solvent, specifically, methanol. Based on the confirmed reactivity and excellent recycling operation of the reagent 1, the methodology has been further extended to a greener dual recycling strategy. By combining the recyclable iodine reagent 1 and silica-supported TEPMO catalyst, a variety of alcohols were effectively oxidized to the desired aldehydes, and the two types of used reagent and catalyst, the iodine 1 and immobilized TEMPO 5, could be separately recovered by an easy workup, and both repeatedly used without any loss of their original activities for at least four cycles.

 Please wait while we load your content...
Please wait while we load your content...