Natural polyphenols as safe alternatives to hydroquinone for the organocatalyzed reduction of dioxygen dissolved in water by diethylhydroxylamine (DEHA)†
Abstract
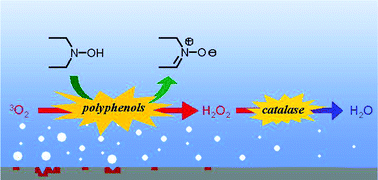
Reduction of dioxygen dissolved in water by diethylhydroxylamine (DEHA) in the presence of hydroquinones or quinones as organocatalysts is investigated with regard to reaction rate and catalyst turnover. Eleven p-benzoquinone derivatives bearing various electron-donating, electron-withdrawing and bulky substituents are studied. The catalytic activity of four benzenediols (hydroquinone, resorcinol, catechol and 4-tert-butylcatechol) and of four benzenetriols (hydroxyquinol, phloroglucinol, pyrogallol and 5-tert-butylpyrogallol) is compared. Mechanistic and kinetic studies indicate that the rate-determining step is the regeneration of hydroquinones through the reduction of the corresponding benzoquinones by DEHA. Then, eight natural polyphenols are tested as alternative catalysts to the potential carcinogen hydroquinone. Four of them (purpurin, myricetin, gallic acid and propyl gallate) exhibit some catalytic activity and gallic acid is shown to be even more efficient than hydroquinone itself. A further improvement of the catalytic system is achieved by adding catalase to disproportionate the generated hydrogen peroxide, which is detrimental for the catalyst. Thus, the oxygen scavenging system DEHA–gallic acid–catalase is found to be not only greener but also more efficient than the current system DEHA–hydroquinone since it gives water instead of hydrogen peroxide as the final reduction product of dioxygen.

 Please wait while we load your content...
Please wait while we load your content...